How Dr. Ehmke Pohl studies protein structures in his Durham University lab
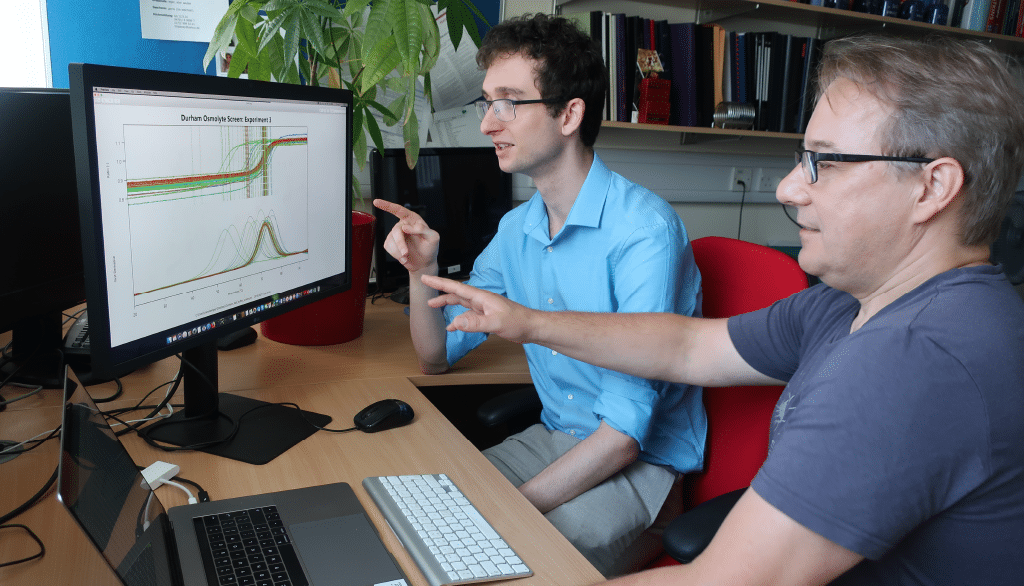
Dr. Ehmke Pohl and Dan Bruce from Durham University pictured.
Structural biologists are experts on the architecture and cellular dynamics of biological macromolecules. Uncovering how a molecule — particularly a protein — assumes its high-ordered structures as well as how its structure influences its function is a universal challenge structural biologists face. To achieve groundbreaking results, structural biologists employ some of the latest cutting-edge tools to better characterize and decipher sometimes very complex molecules.
We took a moment to speak with Dr. Ehmke Pohl from Durham University to gain a better appreciation of the exciting research his lab is doing. Read on to find out more about what we learned from him.
Tell us a little about yourself and the research you are doing at Durham University
I am a structural biologist who uses a range of biophysical methods, in particular, macromolecular crystallography, to determine the 3-dimensional structures of biological macromolecules and their complexes. One focus of the group is centered around proteins that are important to human diseases and have the potential for the development of new drugs. For example, I have been working for many years on the development of potential Tuberculosis booster drugs. 
What is the Virus-X consortium?
The Virus-X consortium is a Horizon 2020 European project by 10 academic groups and 3 small biotechnology companies from 8 countries. The goal of the project is to mine the unexplored genetic diversity of bacteriophages from extreme environments such as Icelandic hot lakes and Atlantic deep-sea vents to discover new enzymes for applications in biotechnology. Using next-generation sequencing, the team has already identified over 50 million novel genes and is developing new tools to identify potentially useful gene products to enter the protein development process that we lead in Durham.
How do you use the information you obtain on the stability of a target protein for your research?
In our key application, macromolecular crystallography, it has been shown repeatedly that although crystallization conditions can never be predicted, the likelihood of obtaining usable crystals is well correlated with protein stability. Therefore, we and others have developed high-throughput screens to routinely measure the stability using 96-well plates. The screens are fully compatible with the Prometheus system from NanoTemper Technologies. By starting with buffer compositions that stabilize the protein, the chances of successfully forming crystals can be significantly increased.
What are the most important features to consider when evaluating a new methodology that you’re interested in adopting into your research workflow?
The ease-of-use and reproducibility are most important. We rely on collaboration in all of our projects hence it is vital that results from day one obtained by one user are the same the next day.
Can you give us examples of how Prometheus and nanoDSF is being tested in any of your current projects?
We used the Prometheus system as part of our Virus-X project. The results were very reproducible and compared well with the traditional thermal shift assay.
What advantages do you see in using a label-free approach to monitoring protein stability?
There are a number of clear advantages (i) sample preparation is easier, (ii) any unforeseeable effects caused by a labeling dye are avoided, and (iii) binding of the dye to hydrophobic pockets can be a problem, particularly, in our drug-design projects.
Why is studying protein stability important or why should researchers care about looking at stability?
Identifying optimal conditions for protein storage, shipment, and use is vital for any commercialization. Even seemingly small differences in pH and buffer conditions can have a profound impact on activity and stability.
When you aren’t working on major research projects or busy in meetings, what do you do as your escape from the lab?
Haha, I wish I had a good answer for that…
Watch the recent Virus-X JOVE video to see how they use 微量差示扫描荧光技术(nanoDSF). Then find out how you can use precisely characterize protein stability with PR (Prometheus).
