优化生物制品以带来更好的临床效果
选择最适合开发的生物制剂意味着要筛选数百、甚至数千种候选物和条件的数据。若您使用糟糕的选拔标准或数据会使得仅仅选择一位候选目标都会变得困难,对于多个候选药物需要进行下游分析和投资的情况,我们需要使用更优的提升临床的可能性。
选择PR Panta可为您的候选生物制剂的多种生物物理属性提供最高质量的数据。同时收集热变性展开、粒径大小和聚集的信息,贯穿整个热变性过程。获得高分辨率,特异性结构域的稳定性表征。了解前所未有的细节,揭示候选物之间的细微差异,因此您可以在管道的早期排除不太稳定的选项
无论您是从事生物制剂配方优化、可开发性还是下游优化工作,PR Panta都能为您的候选目标提供多参数稳定性表征和值得信赖的结果。
PR Panta 综合样品构象、粒径和聚集的数据,帮您更好地表征和筛选候选分子
- 找出候选物之间的细微差别,从而更有效地缩小候选范围
- 花时间寻找答案,而不是做实验
- 从生物制品样品中获得更多的稳定性信息
- 处理当下及未来任何生物项目
找出候选物之间的细微差别,从而更有效地缩小候选范围
当面临成千上万的候选物需要筛选时,即使是结构或条件之间最细微的差异也意味着选择一个最佳候选目标或进行进一步实验之间的差异。PR Panta提供了高分辨率和可重复性的稳定数据,可以显示最微小的变化,从而为管道进展选择最佳候选数据
花时间寻找答案,而不是做实验
PR Panta的操作极其简便。它只需要很少的培训就可以加载样本,直观的软件界面使得数据解释非常简单。Panta提供的高分辨率数据意味着您花更少的时间来破译结果,获得更多的时间来完成结论。
从生物制品样品中获得更多的稳定性信息
PR Panta结合了四种技术——nanoDSF, backreflection, DLS, and SLS——为您提供候选产品的完整稳定性数据。它是唯一一个在整个热变性中收集DLS数据的仪器。单个样品即可提供所有候选生物制剂的胶体和构象稳定性信息,因此您可以用更少的材料和更短的时间获得更多的信息。
处理当下及未来任何生物项目
无论您对生物制剂开发的哪个方面感兴趣——抗体工程、基于蛋白质的治疗设计、配方、ADC或生物仿制药开发——PR Panta都能为您提供所有这些方面的稳定性信息。随着时间的推移,您要解决的问题也在不断演变,Panta将为您提供所有候选目标可靠的稳定性特征。
在整个生物制剂工作流程中监控候选目标的基本属性
由于需要筛选成百上千的候选药物或条件,生物制剂的稳定性数据对于做出良好的管道决策至关重要。关于结构或配方如何影响生物稳定性的信息,可以帮助您将庞大的库缩小到几个-甚至只有一个,以便用于进一步开发。
PR Panta提供高分辨率、多参数的生物物理表征信息。它提供了热稳定性概况以及有关聚集和纯度的信息,因此您可以从同一样品中获得更多数据
阅读更多关于PR Panta如何应用在不同的生物实验场景。
设计具有更高热力学稳定性和更有效折叠的全长抗体、scFv和Fab片段。来自PR Panta的高分辨率数据使您能够看到变体之间最细微的差异,因此您可以构建更稳定的候选版本以向前推进。
使用PR Panta
- 表征构象稳定性
- 确定聚集倾向
- 检测您制剂的均一性
在开发过程中,我们的目标是预测候选药物在整个药物开发管道中是否会成功。通过排除那些物理和化学性质不理想的候选物,可以避免在不理想的候选物上浪费投入的时间。
使用PR Panta
- 确定聚集倾向
- 测量自我和非特定的相互作用
- 表征构象稳定性
处方前研究工作表征了候选分子的基本物理化学性质,这决定了长期储存和递送条件等相关信息。如果一种候选药物在小瓶中不纯净且不稳定,那么它就没有希望成为一种治疗药物。PR Panta进行稳定性分析仅需几微升的样品。
使用PR Panta
- 找到熔解温度和熔化候选目标
- 检测大小聚合
- 确定聚集倾向
- 测量溶液中粒径大小
生物分子的长期生存能力取决于其缓冲环境。评估稳定性属性,以找到最佳缓冲液和辅料,以保持您的治疗在储存期间的稳定性。
使用PR Panta
- 表征构象稳定性
- 确定聚集倾向
- 筛选掉那些影响候选目标稳定和导致聚集的条件
- 寻找能在冷库中保护生物制品的辅料
- 进行加速稳定性研究
一旦选定了一种候选药物,就需要更多的药物进行临床试验,并最终供患者使用。放大和制造会给生产过程引入新的变量,因此继续监控候选产品的稳定性属性非常重要。
使用PR Panta
- 表征热稳定性,确定聚集倾向,并在放大和优化过程中捕获杂质
- 比较不同批次之间的构象和胶体稳定性
- 随着生产过程的发展,监控稳定性曲线的变化
- 将稳定性信息纳入实验设计(DoE)过程
- 在生产过程中引入变更时,执行可比性评估
稳定性信息在候选药物成为专利药物的道路上至关重要。新药开发阶段,在非临床毒理学研究中使用的研究产品需要杂质概况。在BLA阶段,稳定性概况建立适当的复测或有效期指南,长期储存条件,并提供各种环境条件影响的证据。
使用PR Panta
- 评估重组/稀释/混合后热稳定性和粒径的变化
- 从强制降解和光稳定性研究中获得热稳定性和粒径尺寸
看看PR Panta如何为您提供有关候选物质稳定性和纯度的信息
PR Panta配备四种生物制剂表征技术。将nanoDSF、backreflection、 DLS和SLS结合在一个仪器中,您无需运行多次检测即可获得有关样品的更多信息。它们依赖于分子固有的荧光和光散射特性,所以无需任何染料或添加剂。这些技术中收集的信息随着整个热变性进行的过程提供的,让您更深入地了解候选目标的变化过程。
观看视频,了解更多关于这些技术是如何工作的
微量差示扫描荧光技术(nanoDSF)
nanoDSF使用其固有的荧光来测量治疗蛋白的热稳定性。
背反射技术(Backreflection)
背反射技术可测量样品的浊度,可以识别大型无定形聚集体的形成。
动态光散射 (DLS)
动态光散射技术(DLS)被用于评估溶液中粒径的大小。告知蛋白质样本是否折叠良好,或者是否有其他污染物质。
静态光散射技术(SLS)
PR Panta提供多参数稳定性表征,因此您可以获得生物制剂的完整稳定性概况。
想了解更多关于您可获得哪些参数以及它们如何帮助您做出管道决策的信息吗? 请参考下面这份清单:
微量差示扫描荧光技术(nanoDSF)
热变性
Tm (for 330 nm, 350 nm, and ratio)
熔解温度,即蛋白50%展开时温度
通过熔解温度对候选者进行排名是一种行之有效的方法,可以找到最耐热的候选者,被认为是进一步开发的更好选择
Tonset (for ratio)
Temperature at which unfolding begins
More thermostable proteins have higher onset of unfolding; furthermore, candidates with Tonset values close to their Tms are more uniformly folded and therefore more stable
Ea(*parameter derived from data)
展开活化能
驱动生物大分子展开所需的能量越多,这种候选生物大分子就越稳定
Reversibility of unfolding
展开时发生不可逆的点
设计或制定候选材料,以避免或延迟不可逆转的展开和功能丧失
动态光散射 (DLS)
粒径(rh)分析
Tsize (from growth of rH in cumulants fit)
平均粒径尺寸开始上升的温度
Indicates temperature at which protein begins to unfold, thereby indicating how stable it is; higher Tsize indicates greater colloidal stability
平均散射光强度
识别样品浓度是否过高或过低,以进行适当的尺寸分布分析;高质量的DLS数据始于良好的信号质量
累积量或粒径分布/正则化分析
rH
水动力半径反映了溶剂化状态下颗粒的大小
建立候选产品的基准尺寸参数,用于比较配方缓冲液或生产工艺变更前后的差异
PDI
PDI指数代表大小群体的分布,
低PDI值表明制备中没有大聚集体或多个蛋白质群体
自相互作用分析
kD
扩散相互作用可以确定蛋白展开的起始以及对胶体稳定性的影响
Negative kD values indicate propensity towards self-interaction, and therefore greater likelihood of aggregating at the high concentrations required for clinical use
背反射技术(Backreflection)
聚集
Tturbidity
浊度的开始温度,即开始产生>12.5 nm半径大聚集体时的温度
Large, amorphous aggregates contaminate preps and lower safety and efficacy of a biologic sample; aim for high Tturbidity values or no change in turbidity signal when formulating biologics
静态光散射技术(SLS)
分子大小信息
分子量
溶液中所有颗粒的平均分子量
建立候选产品的基准尺寸参数,用于比较配方缓冲液或生产工艺变更前后的差异
自缔合和聚集倾向
Tscattering
散射强度开始改变的温度表明尺寸和聚集增加
Additional parameter for evaluating colloidal and conformational destabilization of a protein; higher Tscattering means a more stable candidate
B22
第二个维里系数用于推断较高浓度下的自聚集行为
Negative B22 values indicate propensity towards self-interaction, and therefore greater likelihood of aggregating at the high concentrations required for clinical use
“用PR Panta对生物制剂进行热变性展开实验,同时检测微观和宏观聚集体,可以非常详细地进行表征。我们的药物发现和开发客户们将受益于这一创新仪器。”
Thomas Schubert, CEO at 2bind, Germany
看看多参数表征如何帮助2bind为他们的候选疫苗选择正确的配方缓冲液
请相信,您是通过精准的、高分辨率的数据,筛选出的正确候选分子
展开转变和粒径大小分析中的微妙细节意味着选择一个候选物或有十几个需要进一步分类的选项之间的区别。解决这些微妙之处还需要可靠的、可重复的候选数据。PR Panta提供高质量的数据,以确保您提供一致,可靠的结果。
通过同时测量提高效率。尽早分享清晰、可操作的结果。
面临大量的候选药物库或大的配方筛选,将最终选择缩小到几个最好的选项是一个挑战。你的下游同事依靠你获得可靠的结果。虽然越多的选择标准可增加了信心,但当它们需要多个实验设置时则会降低效率。
鉴定和区分稳定性
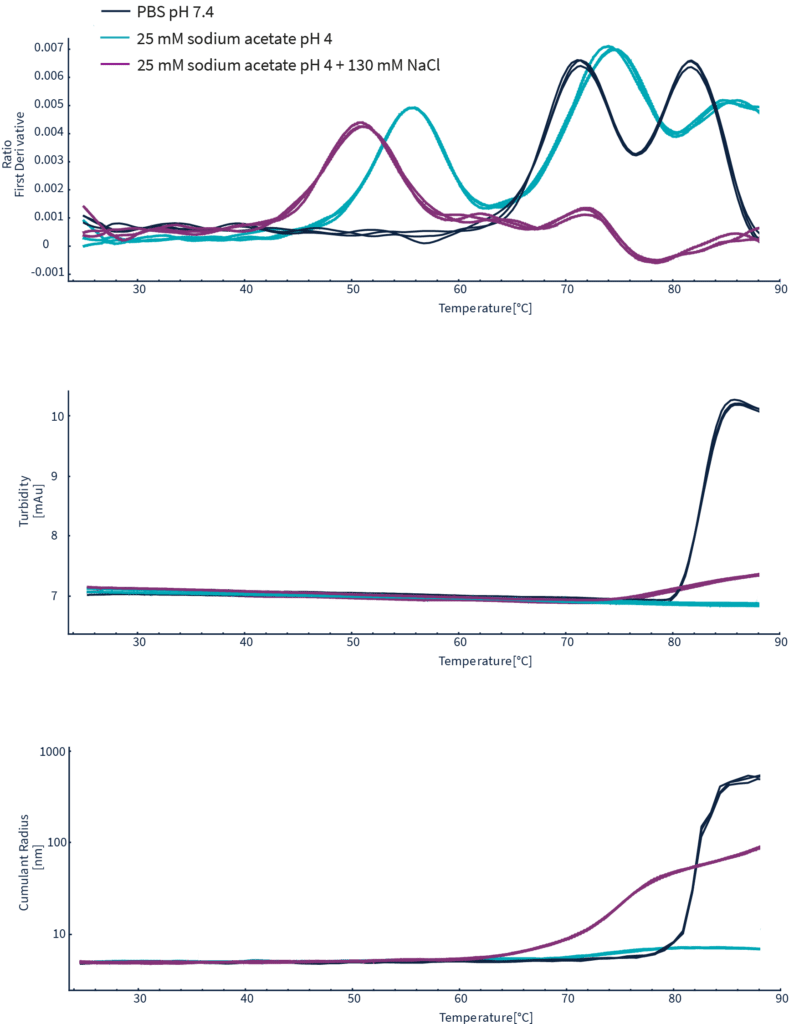
通过Herceptin在三种不同缓冲条件下的整个热变性过程,同时采集测定nanoDSF、DLS和背反射数据。
PR Panta 满足您不断变化的通量需求
大项目,小项目,以及介于两者之间的一切的场景都会出现。更不用说,实验室会随着实验的需求,随着时间的推移而变化。 灵活的吞吐量和各种应用用途使其能够经得起未来的考验,并获得良好的投资回报率。
PR Panta照顾您不断变化的吞吐量需求-为那些较小的项目选择单个毛细管,或毛细管组加载样品更方便更快速适配于的大型项目。并且,您可以获得候选目标的详细稳定性分析,无论他们处于开发管道的哪个阶段。