助您解决亲和力筛选的终极挑战
突破性的光谱位移 (Spectral Shift) 技术
Dianthus是基于微孔板、无微流控系统的亲和力筛选平台。检测在溶液中进行且不依赖于分子量变化,是高难度筛选项目取得成功的绝佳平台。

最重要的靶点和候选药物的亲和筛选总是非常具有挑战性。由于需要固定样品,SPR技术在检测PROTAC三元结合反应,片段化合物库以及固有无序蛋白时面临更大的挑战,而这些应用则是Dianthus所擅长的。
基于光谱位移(Spectral Shift)和温度依赖的荧光强度变化(TRIC)这两种亲和力检测技术,Dianthus可以提供高质量的数据,灵敏检测更多真正的结合分子,并且仅需较少的方法优化即可为您提供真实样品检测结果。
在高难度亲和力筛选项目中获得成功
用可信的结果推进筛查实验
当面对具有挑战性的筛选时,大多数生物物理方法最多只能提供低质量的数据,有些甚至根本不起作用。而 Dianthus 可提供高信噪比的数据,特别是针对计算亲和常数时,可消除hits的任何不确定性,用可靠的数据决策您的实验。
从实际样品中获得可行的结果,省去在分析开发上花费太多时间
一些亲和筛选方法只能通过纯样本生成良好的数据,这样会拖延项目,给筛选团队带来了很大的压力。使用 Dianthus,您可以从实际样品中获得高质量的数据,免受聚集体,杂质或沉淀物的阻碍。
依靠具有敏感度的生物物理技术来识别更有意义的hits
为了避免忽略有意义的hits,通常使用多种方法进行筛选——但这使得这个过程既漫长又费力。而通过 Dianthus 的检测可以到非常微妙的光谱变化,您可以避免因假阴性结果而错过获得更多的有效结合数据。
克服其他技术遇到的常见问题
同其他科学家一样,您在攻关筛选项目的过程中一定也会被相同的问题所困扰。Dianthus让您轻松专注于自己的研究项目,无需再担心这些问题。
测量最广泛的亲和力范围
Dianthus 检测到广泛的结合亲和力-从皮摩尔到毫摩尔-可以捕获非常强和弱的结合。
可在溶液中表征,无需固定
可在接近天然条件下分析相互作用,特别是在处理具有挑战性的目标。由于测量是在溶液中完成的,因此不必担心对目标结合位点产生负面影响或缺乏对平衡条件的控制。
消耗少量的目标和化合物
一点一滴都很重要。节省昂贵的样品和库化合物意味着我们可以做更多的筛选实验或项目
自动化处理亲和筛选实验
可与许多自动化解决方案兼容,获得数小时不间断和无人值守的操作
Dianthus系列,为您移除药物研发中的障碍
当药物研发过程涉及到以下复杂的亲和力筛选难题时,配备 Dianthus 可以助您更高效地完成 hits 识别和 leads 优化。
PROTACs 等小分子蛋白质降解剂
蛋白降解剂研发的流程复杂、步骤繁多。因此,在每个阶段选择合适的生化及生物物理方法能够更快速有力地推动候选药物的研发进度并推向市场。
Dianthus能够克服二元和三元复合物表征以及协同性评估中的各种挑战。
了解Dianthus在PROTAC中的应用
片段化合物库
挑战
如果您的方法需要显著的质量变化来测量相互作用,那么您将很难从片段库中识别苗头化合物(hits),因为通常它们的分子量非常低。
解决方法
使用Dianthus,您可以自信地从片段库中识别hits,因为筛选测定亲和力所使用的技术,不依赖质量变化。
此外,由于 Dianthus 可检测的亲和力强度范围广泛,可以帮您识别片段和蛋白 mM 级弱亲和力的 hit;当片段形成以低 nM 亲和力结合的较大化合物时,它对 lead 的优化也展现出很大的优势。
固有无序蛋白 (IDPs) 和其他易于聚集的靶点
-
挑战
因为IDPs不会折叠成均匀的3D结构,所以您会看到由非原生分子间相互作用引发的聚集。除此之外,您还需要关注其他方法所需的高浓度所导致的聚集。 -
解决方法
使用Dianthus, 不需要像使用 ITC 那样依赖高浓度。若聚集发生在低浓度下,Dianthus 会区分结合和聚集,这样可以更好地理解分子的行为。 -
挑战
若方法需要固定,这很容易扰乱IDPs的构象平衡 -
解决方法
Dianthus可在溶液中测量,因此构象平衡不会像SPR技术那样需要固定-那样有被破坏的危险
Dianthus是您自始至终值得信赖的筛选平台
Hits 的发现
更快地发现 hits,是提高药物研发效率的最关键步骤。在 fragment 或小分子单点筛选中,利用 DI,您可以快速发现 hits,并进行确认。
先导化合物 (Leads) 确认
DI 可以生成简单、易于解释的亲和力排序表和注释,帮助您快速确定合适的候选分子,并马上开始先导化合物 (leads) 的优化,而不必在强、弱活性分子的排序上花费过多时间。
先导化合物 (Leads) 优化
一旦确认完成,下一步就需要提高化合物的特异性、选择性以及效价。使用 DI 可以确认那些化合物的亲和力有无变化。这一数据与您的 ADME,毒理以及 PK/PD 结果一起,可以帮助您开发最有潜力的候选药物分子。
兼具两种生物物理技术的平台验证hits
多种生物物理方法的正交验证是所有主要筛选活动的必备条件。用一种方法找到的结果被另一种方法发现是聚集体造成的假阳性的情况并不罕见。正交验证通过确认hits和发现其他方法错过的药物质量潜力化合物来降低项目风险。研究人员选择Dianthus进行正交验证,确认并收集额外的化合物性质,以增加了解哪些苗头化合物(hits)能够进入到苗头化合物至先导化合物(hit-to-lead)的步骤。
两种生物物理检测方法确保您成功完成筛选
测量不同类型分子之间具有挑战性的相互作用有时需要不同的方法。所以在同一仪器中使用两种不同的模式可以帮助你涵盖你遇到的所有类型的交互验证。Dianthus具有两种生物物理模式-光谱位移和温度相关强度变化(TRIC) -帮助您测量具有挑战性的分子相互作用的强度。
-
光谱位移技术 Spectral Shift
-
TRIC
光谱位移技术 Spectral Shift
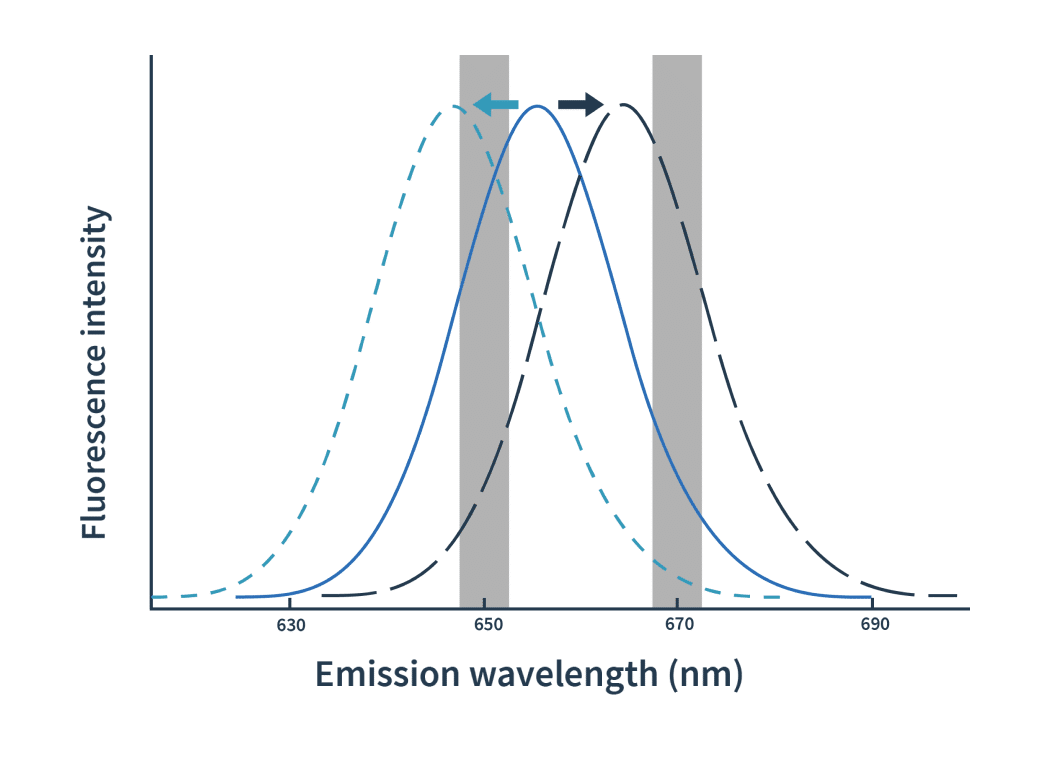
Dianthus是第一个使用光谱位移技术的亲和力筛选平台。光谱位移的概念并不新鲜,但Dianthus是第一个使用它来推导亲和常数的平台。
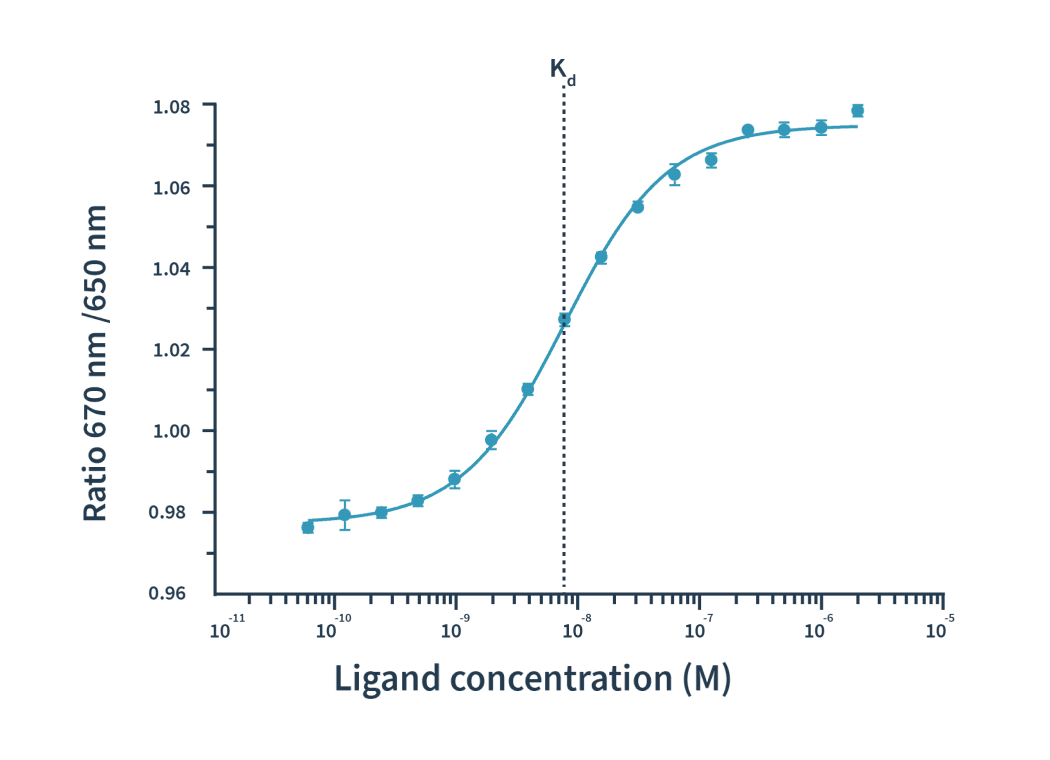
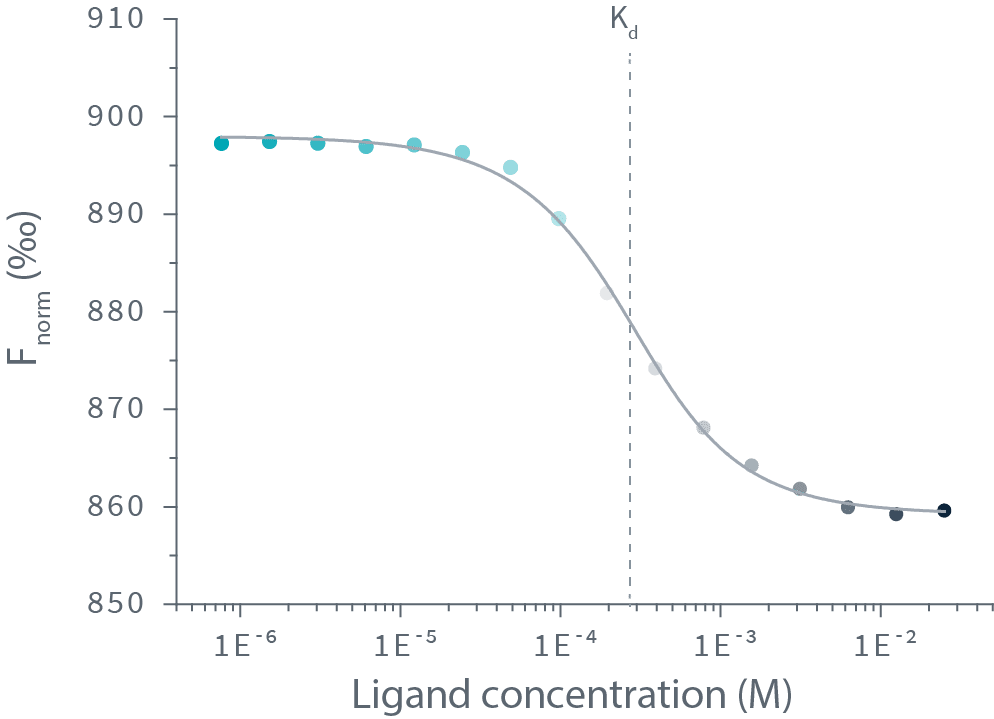
实验流程非常简单:我们对靶标分子进行荧光标记,然后将其与一系列梯度稀释的配体分子等量混合。以590nm激发光对混合物进行荧光激发后,配体与靶标分子的结合可通过发射光谱的蓝移或红移得到检测。Dianthus在等温条件下精确检测650 nm和670 nm双波长的发射光,因而能够准确测到极细微的光谱位移。
Then the Kd is calculated by plotting the ratios of the fluorescence intensities from the two wavelengths against the ligand concentration.
TRIC
使用TRIC这项历时 10 年验证的成熟技术对光谱位移进行补充
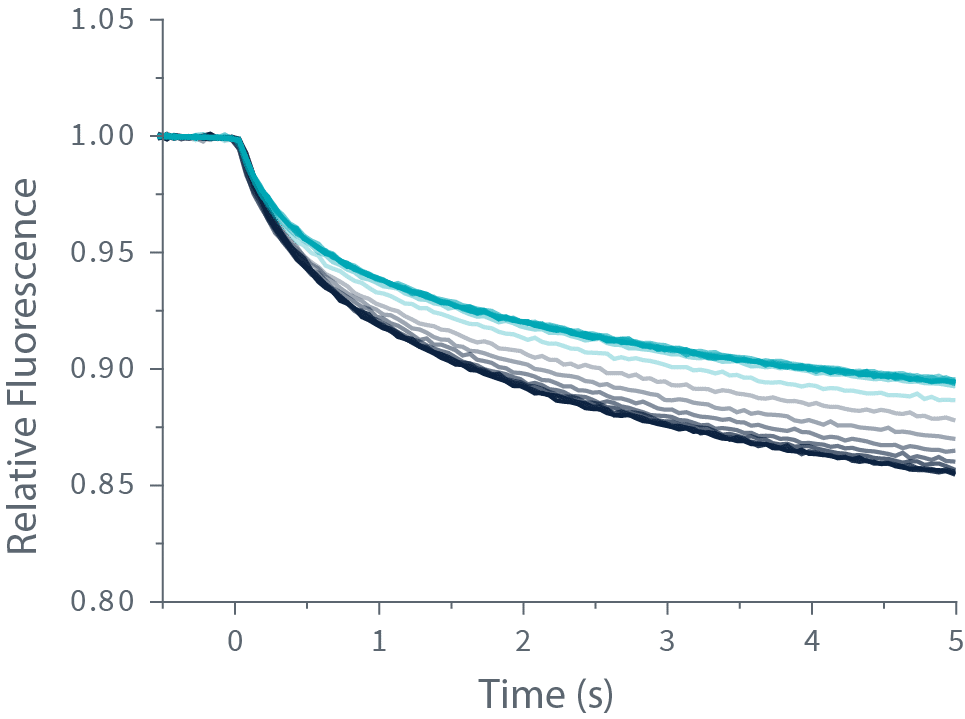
TRIC技术是通过对靶标分子进行荧光标记,并使其与配体分子混合来定量检测分子间相互作用。随后,通过激光,在溶液中制造一个精确而短暂的温度变化,可放大由配体与靶标分子结合引起的荧光强度变化。
The change in fluorescence is measured and plotted against the ligand concentration to obtain the dissociation constant or Kd.

将Dianthus纳入到您现有的自动化解决方案中
Dianthus是一个基于孔板的无微流控筛选平台,可以通过gRPC框架轻松集成到任何自动化设置中。由于无需定期维护,您的项目不会因停机而延迟。但当您需要它的时候,Dianthus则随时待命——不间断,24小时/7天。
挑选有价值的hits进行优化
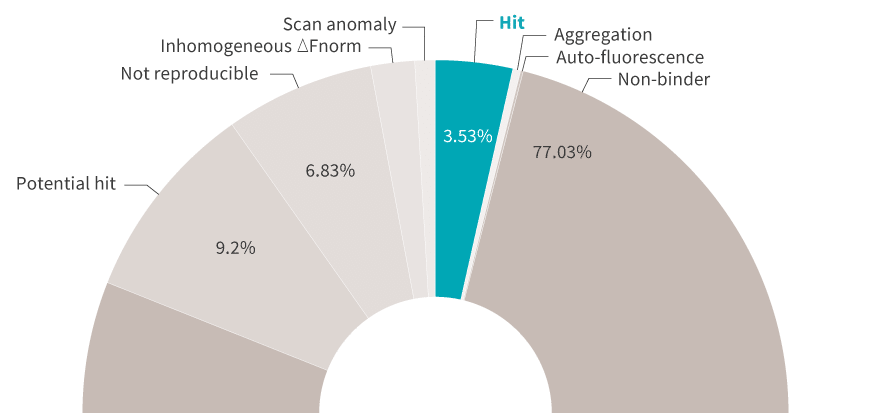
产生结果很好,若可从结果中获得自动化的、可操作的见解就更好了。DI.筛选分析软件为您提供筛选摘要以及易于解释的排名表和柱状图。由于Kds是根据高信噪比的数据计算的,因此您可以确定哪些候选人值得继续跟进。