Optimize your biologic therapeutics for better clinical outcomes
Selecting the best biologic for development means sifting through data on hundreds, or even thousands, of candidates and conditions. Poor selection criteria or bad data make it difficult to choose just one candidate for advancement; but downstream assays on multiple candidates require time and investment on therapeutics that will never see the clinic.
Prometheus Panta provides the highest quality data on multiple biophysical attributes of your biologics candidates. Collect information on thermal unfolding, particle sizing, and aggregation simultaneously, throughout the entire thermal ramp. Get high-resolution, domain-specific stability characterization. See unprecedented detail that unveils subtle differences between candidates, so you eliminate less stable options earlier in the pipeline.
Whether you’re working in biologics formulation optimization, developability, or downstream optimization, count on Prometheus Panta to provide multi-parameter stability characterization and trustworthy results for your candidate molecules.
Prometheus Panta combines thermal unfolding, particle sizing, and aggregation for superior characterization of candidate molecules
- Find subtle differences between candidates to narrow down your pipeline more efficiently
- Spend time getting answers instead of setting up experiments
- Get more stability information out of your biologics samples
- Tackle any biologics project that comes your way
Find subtle differences between candidates to narrow down your pipeline more efficiently
When you have thousands of candidates for stability screens, even the subtlest difference between constructs or conditions means the difference between selecting a single, best candidate or conducting further experiments. Prometheus Panta provides stability data with a high degree of resolution and reproducibility, revealing the slightest changes so you select the best candidate for pipeline progression.
Spend time getting answers instead of setting up experiments
Getting started with the Prometheus Panta is pain-free. It requires very little training to get your samples loaded, and the intuitive software interface makes data interpretation straightforward. The high-resolution data Panta provides means you spend less time deciphering results and more time drawing conclusions.
Get more stability information out of your biologics samples
Prometheus Panta incorporates four technologies – nanoDSF, backreflection, DLS, and SLS – to give you a complete stability profile of your candidates. And it’s the only instrument that collects a DLS profile throughout the entire thermal ramp. A single sample provides colloidal and conformational stability information about all your biologics candidates, so you get more information with less material, and in less time.
Tackle any biologics project that comes your way
Whatever aspect of biologics development you’re interested in – antibody engineering, protein-based therapeutic design, formulation, ADC or biosimilar development – Prometheus Panta gives you stability information on all of it. As the questions you address evolve over time, Panta is there to give you reliable stability characterization on all your candidates.
Monitor essential attributes for candidate selection throughout your biologics workflow
With hundreds to thousands of candidates or conditions to screen, stability data on your biologics is critical for making good pipeline decisions. Information on how a construct or formulation impacts the stability of your biologic helps you narrow down huge libraries to just a few – or even just one – for further development.
Prometheus Panta provides high-resolution, multi-parameter biophysical characterization information. It gives you a thermal stability profile alongside information about aggregation and purity, so you have more data to work with from the same sample.
Read more about how Prometheus Panta helps with different experiments for biologics.
Engineer full-length antibodies, scFv, and Fab fragments with higher thermodynamic stability and more efficient folding. High-resolution data from Prometheus Panta enables you to see even the most subtle differences between variants, so you build more stable candidates to move forward.
Use Prometheus Panta to
- Characterize conformational stability
- Determine aggregation propensity
- See how uniform your preps are
During developability, the goal is to predict whether candidates will be successful throughout the drug development pipeline. Eliminate candidates with undesirable physical and chemical properties early on, so you avoid spending time on poor candidates.
Use Prometheus Panta to
- Determine aggregation propensity
- Measure self- and non-specific interactions
- Characterize conformational stability
Preformulation work characterizes basic physiochemical properties of a candidate molecule, which dictate relevant information like long-term storage and delivery conditions. A candidate isn’t promising as a therapeutic if it isn’t pure and stable in the vial. Stability profiling with Prometheus Panta informs on many stability characteristics with just a few microliters of sample.
Use Prometheus Panta to
- Find melting temperature and melting onset of your candidates
- Detect large and small aggregates
- Determine aggregation propensity
- Measure size of particles in solution
Long-term viability of a biologic molecule is dependent on its buffer environment. Evaluate stability attributes to find the best buffers and excipients to keep your therapeutic stable during storage.
Use Prometheus Panta to
- Characterize conformational stability
- Determine aggregation propensity
- Screen out conditions that destabilize your candidate and cause aggregation
- Find excipients that protect your biologic in cold storage
- Perform accelerated stability studies
Once you select a candidate, a lot more of it is required for clinical tests, and eventually for patient use. Scale-up and manufacturing introduce new variables to the production process, and it’s important to continue monitoring the candidate’s stability attributes.
Use Prometheus Panta to
- Characterize thermal stability, determine aggregation propensity, and catch impurities during scale-up and optimization of processes
- Compare conformational and colloidal stability profiles between batches
- Monitor changes in stability profiles as production processes evolve
- Incorporate stability information into Design of Experiment (DoE) processes
- Perform comparability assessments as changes are introduced to the production process
Stability information is critical along a candidate’s road to becoming a patented drug. At the IND stage, impurity profiles are required for investigational products used during non-clinical toxicological studies. At the BLA stage, stability profiles establish appropriate retest or expiration guidelines, long-term storage conditions, and provide evidence of the effect of various environmental conditions.
Use Prometheus Panta to
- Assess changes to thermal stability and particle sizing after reconstitution/dilution/admixing
- Get thermal stability and particle sizing from forced degradation and photostability studies
See how Prometheus Panta gives you information about your candidates’ stability and purity
Inside a Prometheus Panta are four technologies for biologics characterization. With nanoDSF, backreflection, DLS, and SLS combined in a single instrument, you get more information about your samples without running multiple assays. They rely on the intrinsic fluorescence and light scattering properties of your molecules, so you don’t need any dyes or additives. And information from these technologies is collected along the entire thermal ramp, giving you more insight into your candidate’s behavior.
Watch these videos to learn more about how these technologies work.
nanoDSF
nanoDSF measures the thermal stability of your therapeutic proteins using their intrinsic fluorescence.
Backreflection
Backreflection measures the turbidity of your sample, that is, it identifies the formation of large amorphous aggregates.
Dynamic Light Scattering (DLS)
Dynamic Light Scattering (DLS) is used to assess the size of particles in solution. It tells you whether your protein samples are well-folded, or whether there are other contaminating species.
Static Light Scattering (SLS)
Get high-quality protein stability information without using a lot of sample
Prometheus Panta uses 10 µl of sample in capillaries to measure your samples. The system has a small benchtop footprint, and allows switching between individual capillary handling, or 24-capillary chips for loading from 384-well plates.
Prometheus Panta provides multi-parameter stability characterization, so you get a full stability profile of your biologics.
Want to know more about which parameters you get, and how they help you make pipeline decisions? Here’s the list:
nanoDSF
THERMAL UNFOLDING
Tm (for 330 nm, 350 nm, and ratio)
Melting temperature, or point at which 50% is unfolded
Ranking candidates by melting temperature is a well-established method for finding most thermostable candidates, considered better selections for further development
Tonset (for ratio)
Temperature at which unfolding begins
More thermostable proteins have higher onset of unfolding; furthermore, candidates with Tonset values close to their Tms are more uniformly folded and therefore more stable
Ea(*parameter derived from data)
Activation energy of unfolding
The more energy required to drive unfolding of a biologic, the more stable that candidate is
Reversibility of unfolding
Point at which irreversibility happens when unfolding
Engineer or formulate candidates to avoid or delay irreversible unfolding and loss of function
Dynamic Light Scattering (DLS)
SIZE ANALYSIS
Tsize (from growth of rH in cumulants fit)
Temperature at which average particle size begins to increase
Indicates temperature at which protein begins to unfold, thereby indicating how stable it is; higher Tsize indicates greater colloidal stability
Average scattering intensity
Identifies if concentration is too high or low for proper size distribution analysis; high-quality DLS data starts with good signal quality
CUMULANT OR SIZE DISTRIBUTION/REGULARIZATION ANALYSIS
rH
Hydrodynamic radius shows size of particle in solvated state
Establishes baseline sizing parameter about a candidate, used to compare before and after changes to formulation buffer or production process
PDI
Polydispersity index represents distribution of size populations
Low PDI values indicate prep is free of large aggregates or multiple protein populations
SELF-INTERACTION ANALYSIS
kD
Diffusion interaction identifies onset of unfolding and impact on colloidal stability
Negative kD values indicate propensity towards self-interaction, and therefore greater likelihood of aggregating at the high concentrations required for clinical use
Backreflection
AGGREGATION
Tturbidity
Onset temperature of turbidity, or large aggregates >12.5 nm radius
Large, amorphous aggregates contaminate preps and lower safety and efficacy of a biologic sample; aim for high Tturbidity values or no change in turbidity signal when formulating biologics
Static Light Scattering (SLS)
SIZING INFORMATION
Molecular weight
Average molecular weight of all particles in solution
Establishes baseline sizing parameter about a candidate, used to compare before and after changes to formulation buffer or production process
SELF-ASSOCIATION AND AGGREGATION PROPENSITY
Tscattering
Temperature at which scattering intensity begins to change, indicating increased size and aggregation
Additional parameter for evaluating colloidal and conformational destabilization of a protein; higher Tscattering means a more stable candidate
B22
Second virial coefficient used to extrapolate self-association behavior at higher concentrations
Negative B22 values indicate propensity towards self-interaction, and therefore greater likelihood of aggregating at the high concentrations required for clinical use
“The simultaneous detection of micro- and macro-aggregates with thermal unfolding of biologics with Prometheus Panta allows for very detailed characterization. Our drug discovery and development customers will benefit from this innovative instrument.”
Thomas Schubert, CEO at 2bind, Germany
See how multi-parameter characterization helped 2bind pick the right formulation buffer for their vaccine candidate
Be confident you’re choosing the right candidates with precise, high-resolution data
Subtle details in unfolding transitions and sizing analysis means the difference between selecting one candidate or having a dozen options that require further triage. Resolving those subtleties also requires reliable, reproducible data on your candidates. Prometheus Panta supplies high-quality data to ensure you deliver consistent, dependable results.
Become more efficient with simultaneous measurements. Share clear, actionable results sooner.
With vast libraries of candidates or large formulation screens, it’s a challenge to narrow down your selection to just a few best options. Your colleagues downstream rely on you for dependable results. More selection criteria boost confidence in your choice, but reduce efficiency when they require multiple experimental set-ups.
Identify and differentiate stability behavior
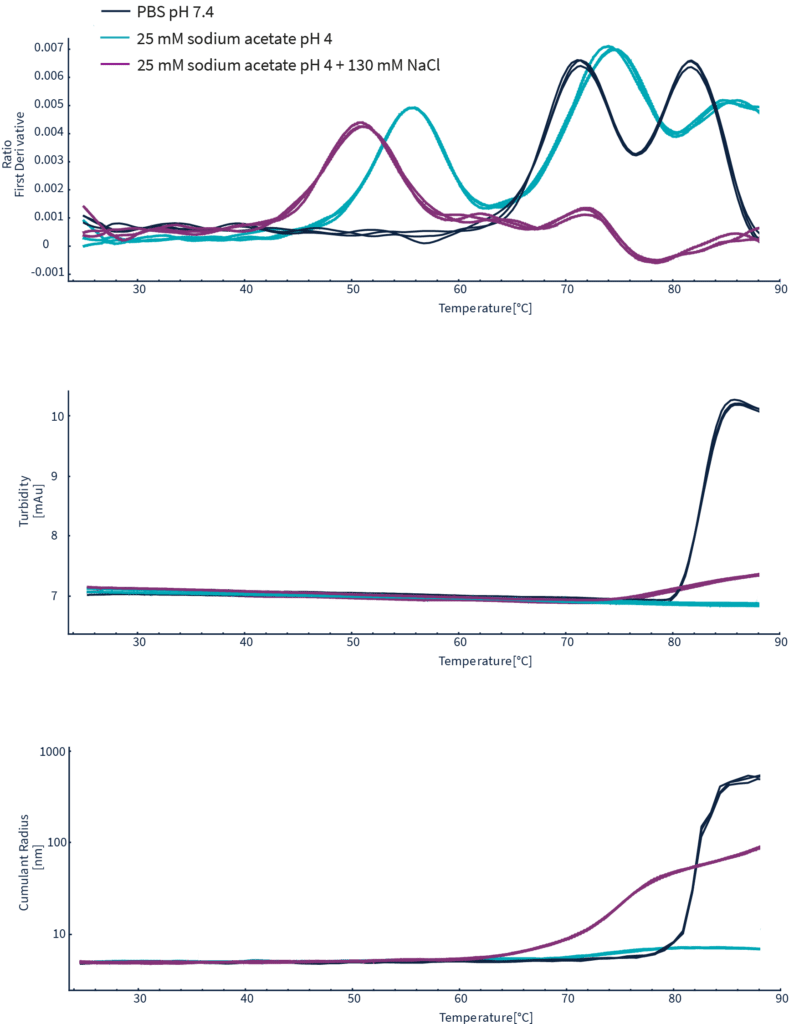
Simultaneous acquisition of nanoDSF, DLS, and backreflection measurements collected throughout the entire thermal ramp for Herceptin in three different buffer conditions.
Prometheus Panta takes care of your changing throughput demands
Large projects…small projects…and everything in between will come your way. Not to mention, the experimental needs of your laboratory change over time. Flexible throughput and a variety of application uses enable future-proofing, and reflect a good ROI.
Prometheus Panta takes care of your changing throughput needs — choose single capillaries for those smaller projects or capillary chips to make loading samples more convenient and faster for the larger ones. And you have the ability to get detailed stability profiling of your candidates, no matter where they are in the development pipeline.