Use Monolith — the only way to get binding affinities in solution using very minimal sample
-
Monolith
- Technology
- Software
- Consumables
- Assay Tools
Directly measure the strength of molecular interactions in solution, without the need for immobilization. Characterize nearly all types of molecular interactions — even the most challenging ones. With ultra-low sample consumption and versatile capabilities, Monolith is the best solution for your binding affinity experiments.
Introducing Monolith X with breakthrough Spectral Shift Technology
With Monolith X, you’ll get Spectral Shift and MST — two biophysical modalities to help cover all the types of interactions you encounter. You’ll enjoy high-quality results without spending your time on assay development and finally perform experiments without worrying about sample aggregation or impurities.
Many great ways to use Monolith
-
Work with challenging interactions that are difficult for SPR
-
Validate your SPR results with an immobilization-free method
-
Use a versatile tool to tackle a wide variety of projects
Work with challenging interactions that are difficult for SPR
Work with challenging interactions that are difficult for SPR
For one reason or another, SPR has a difficult time analyzing challenging binding events no matter how many times you’ve tried to develop an assay. When you find yourself in these challenging situations, turn to Monolith to help.
Immobilization prevents you from getting a Kd
Suboptimal immobilization or regeneration conditions in SPR negatively impact ligand-binding activity. Because Monolith measures binding in-solution under controlled equilibrium conditions, it will help you handle more challenging ligands, like IDPs with complex conformational dynamics.You experience non-specific binding between the analyte and matrix
Since SPR doesn’t differentiate between binding of an analyte to an immobilized ligand or to the matrix on a sensor, you’ll find yourself doing further testing to recognize and avoid non-specific binding. With Monolith, there’s no need to test for non-specific binding since measurements are done in solution.You’re unable to easily resolve high affinity interactions
It’s so difficult to assess high affinity interactions using SPR. Why? Because these types of interactions have very slow dissociation rates and since SPR uses this information to derive binding data — getting a result could take forever. With Monolith, binding is measured directly, so you don’t have to wait.You’re dealing with covalent interactions
Studying covalent binders with SPR is very complex. Because Monolith measures interactions in solution, it’s easy to measure covalent interactions — there’s no need to figure out how to regenerate biosensors.Validate your SPR results with an immobilization-free method
Validate your SPR results with an immobilization-free method
It’s common practice to validate your results with more than one technique because you want to be confident that the results are real. Monolith, with its immobilization-free measurement, is the perfect orthogonal tool for SPR users. It removes the immobilization bias and helps to confirm your results, identify false positives, or find binding partners that your primary assay missed.
Use a versatile tool to tackle a wide variety of projects
Use a versatile tool to tackle a wide variety of projects
Rely on Monolith’s versatile capabilities to execute immobilization-free experiments quickly and efficiently. Tackle projects that involve almost any molecule, buffer composition, or binding strength, all while consuming only a small amount of sample.
Get binding affinity data for almost any type of molecule
Work with almost any molecule including IDPs, membrane proteins, large protein complexes, PROTACS, small molecules or ions.
Capture mass and size-independent measurements
Evaluate results independently of size and mass differences in binding partners.
Utilize a myriad of assay buffer formulations
Analyze the binding of purified and crude samples without worrying about interference from buffer additives like detergents.
Quantify low and high binding affinities
Measure a broad range of binding affinities, from pM to mM, allowing you to detect strong and weak binders.
Get more than just a Kd
Scientists use Monolith to study binding stoichiometry* and thermodynamic parameters*, assess relative affinities with competition assays, and characterize binding cooperativity*.
*Requires offline data handling, not supported by Monolith software
You’ll love that Monolith is maintenance-free and easy to use
Maintenance-free
Because Monolith doesn’t use fluidics, doesn’t require warm-ups, calibrations, washes, cleaning or flushing in between runs, you are able to run assays whenever you want.
Easy-to-use software
You’ll be ready to run experiments independently after only one day of training. Monolith’s software takes you through step-by-step instructions on how to prepare and run your assay – making it a straightforward experience for anyone on your team.
“It has proved a valuable tool for characterizing small molecule-protein and protein-protein interactions, as well as for the study of protein aggregation concentration determination. There is very good agreement with other technologies such as Surface Plasmon Resonance (SPR) and Isothermal Titration Calorimetry (ITC), and we are particularly appreciative of this new technology because of the extremely low protein consumption and relatively short time required for the assay setup.”
Life is full of possibilities — so is your research with Monolith
Monolith X
with Spectral Shift and MST technologies
Monolith X is equipped with Spectral Shift and MST technologies and provides you high quality data with little to no assay development.
Monolith
with MST technology
Monolith comes with MST and enables you to measure high affinity interactions in the pM range, work label-free, or use GFP-tagged proteins.
Learn more about the technologies in Monolith
Monolith comes with two biophysical modalities — Spectral Shift and MST — that help you measure the strength of your molecular interactions. Experiments start by labeling one molecule with a fluorophore*, then mixing a fixed amount of it with a dilution series of the binding partner. Samples are then loaded into capillaries and are placed into the instrument for the measurements to start.
*Alternatively, the intrinsic fluorescence of tryptophan can be used for MST.
-
Spectral Shift
-
MST
Spectral Shift Technology
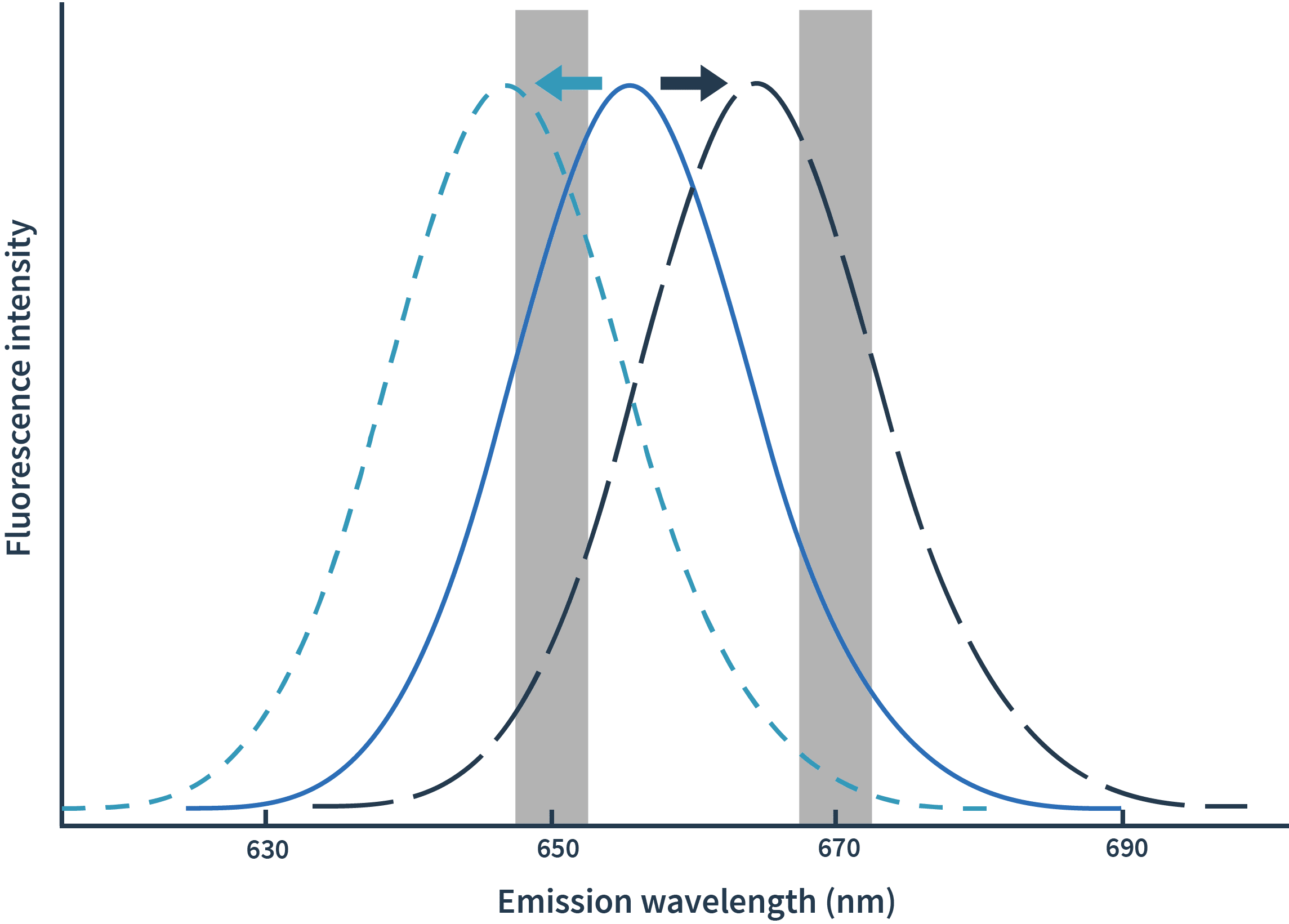
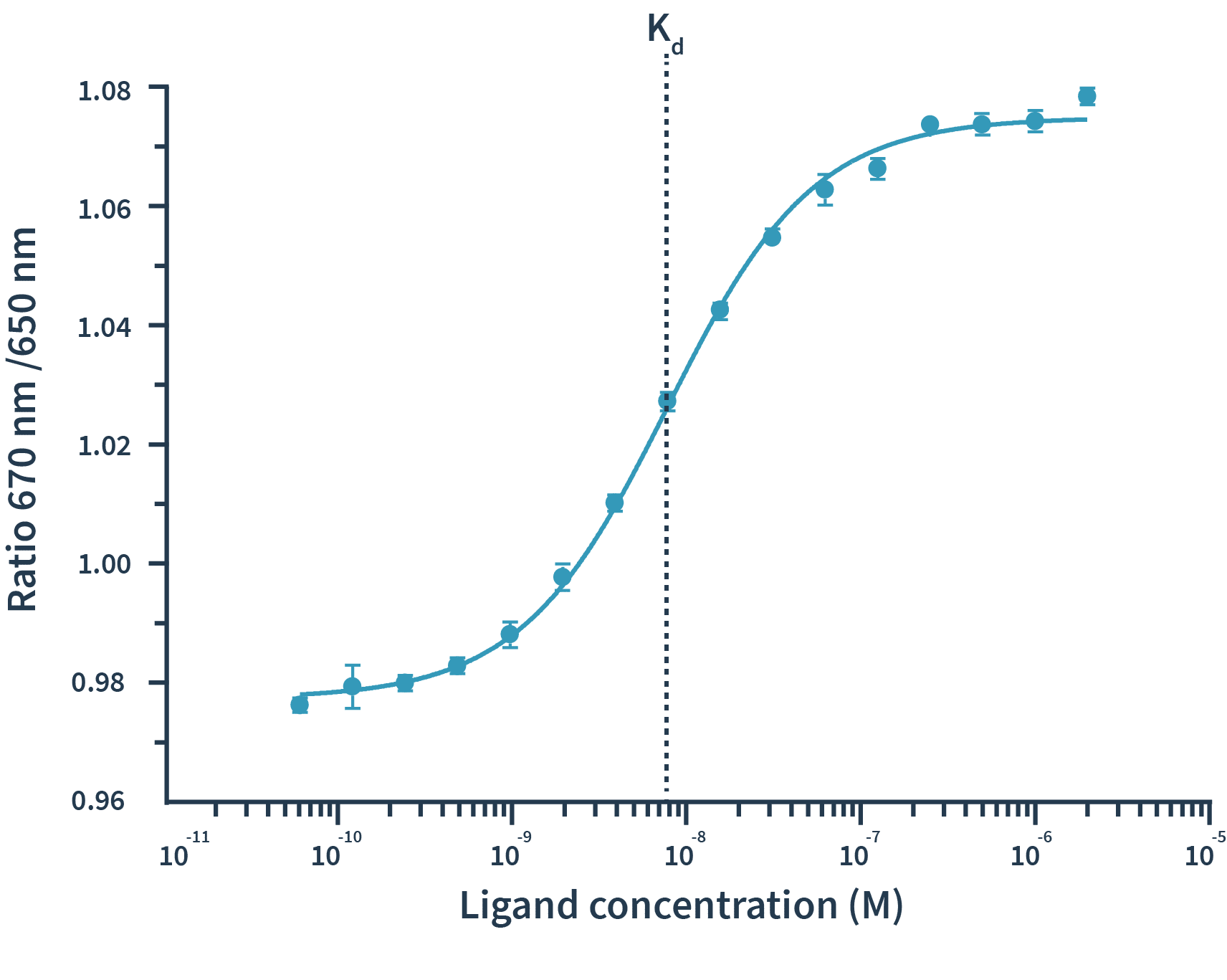
With Spectral Shift measurements, binding events are detected as shifts of spectra towards either blue or red. The detection of spectral shifts is achieved through dual-wavelength detection at exactly 650nm and 670nm in an isothermal environment — this allows Monolith X to detect subtle shifts with high precision.The Kd is then calculated by plotting the ratios of the fluorescence intensities from the two wavelengths against the concentration of the binding partner.
MST Technology
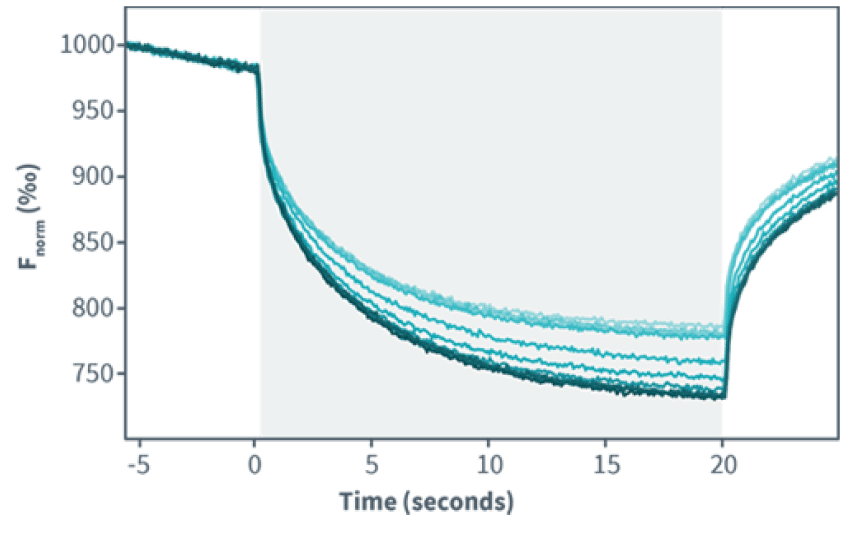
MST measures variations in fluorescence intensity caused by ligand binding while a very precise and brief laser-induced temperature change is applied.The change in fluorescence is then plotted against the concentration of the binding partner to obtain the dissociation constant or Kd.

Feel confident your experiments will run smoothly with software that’s smart
Most software begins with loading a sample and starting the measurement. Monolith’s software is built differently — not only does it provide step-by-step experimental planning and assay setup guidelines, but it also provides immediate feedback on assay optimization based on the results. In addition, you have the option to merge data sets and group them for comparison purposes, to get results that are reported with presentation-worthy data and publication-ready figures.
Get great results with tailor-made consumables
Monolith capillaries are manufactured in a state-of-the-art facility and rigorously tested. For the highest success, pair the capillaries with a Monolith Protein Labeling Kit to get the highest quality data and ultimately the best outcome.

Use additional resources to reach project success more efficiently
Protein Labeling Assistant
With the online and interactive Protein Labeling Assistant, you’ll get the best labeling recommendations for proteins and instrument optics.
Buffer Exploration Kit
Make buffer screen less time-consuming with the Buffer Exploration Kit, a 96-well plate loaded with ready-to-use buffers.