Tackle your most challenging affinity screenings with Dianthus
Now with breakthrough Spectral Shift technology
-
Dianthus
- Applications
- Technology
- System
- Software
- Consumables
Dianthus is a plate-based and microfluidics-free affinity screening platform. Measurements are in solution and mass-independent — ideal for succeeding at demanding screening campaigns.

It’s not surprising that the most important drug candidates and targets are super challenging when it comes to affinity screening. Immobilization-dependent SPR struggles with affinity screening campaigns for applications involving PROTAC binary and ternary complexes, fragment libraries, and intrinsically disordered proteins. These are precisely the applications where Dianthus excels.
Now with Spectral Shift and TRIC, two methods for measuring molecular interactions, Dianthus delivers high-quality data, has the sensitivity to detect more true binders, and requires less assay development to give you results from real-life samples.
Finally find success with your challenging affinity screening campaigns
Move your screening campaigns forward with results you can trust
When faced with challenging screenings, most biophysical methods deliver poor-quality data at best, and some just don’t work. Dianthus delivers data with high signal-to-noise ratios that remove any uncertainty about your hits, especially when calculating affinity constants. So you trust your data and your decision-making.
Get actionable results from real-life samples without spending too much time on assay development
Some affinity screening methods only generate good data with pure samples, which delays projects and puts a lot of pressure on screening teams. With Dianthus you get high-quality data from real-life samples, so aggregates, impurities, or precipitates won’t hold you back.
Rely on a biophysical modality that has the sensitivity to identify more meaningful hits
To avoid overlooking meaningful hits, you screen with multiple methods — which makes the process long and laborious. Because Dianthus detects very subtle spectral shifts, it’s all you need to identify more binders and fewer false negatives.
Beat common concerns encountered with other technologies
No matter what screening campaign you’re tackling, you are bound to encounter common issues that worry most scientists. Relax and focus on your research knowing that with Dianthus, you don’t have to fret about them.
Measure the broadest range of affinities
Dianthus detects a wide range of binding affinities — picomolar to millimolar — so you catch very strong and weak binders.
Characterize in solution, no immobilization required
Analyzing interactions in close-to-native conditions is ideal, especially when dealing with challenging targets. Never worry about negatively impacting your target’s binding site or lacking control over equilibrium conditions, since measurements are done in solution.
Consume small amounts of target and compounds
Every little bit counts. Saving on costly samples and library compounds means you can do more screening campaigns or projects.
Automate your affinity screening campaigns
Get hours of uninterrupted and unattended operation with a format that’s compatible with many automation solutions.
Lean on Dianthus when your affinity screening campaigns encounter roadblocks
Don’t give up just yet when you’re faced with a complicated affinity screening campaign. Armed with Dianthus, you have an opportunity to keep your project moving forward when your hit identification or lead optimization involves any of these molecules.
PROTACs and other small molecule protein degraders
Development of protein degraders is a multi-step and complex process. Selecting the appropriate biochemical and biophysical methods for each step is crucial to move the drug candidates down the pipeline and to market faster and with confidence.
Dianthus resolves the challenges presented by the characterization of binary and ternary complexes and the determination of cooperativity.
Learn how to use Dianthus for PROTACs
Fragment libraries
Challenge
Because your method requires a significant change in mass to measure interactions, it’s difficult to identify hits from a library of fragments due to their very low molecular weight.
Solution
With Dianthus, you identify hits from fragment libraries with confidence because measurements are mass-independent.
And, because Dianthus detects a broad range of affinity strengths, it’s useful for hit identification when fragments bind to proteins with mM affinity and lead optimization when fragments grow to form larger compounds that bind at low nM affinities.
Intrinsically disordered proteins (IDPs) and other aggregation-prone targets
-
Challenge
Because IDPs don’t fold into a homogeneous 3D structure, you see aggregation triggered by non-native intermolecular interactions. On top of that, you are concerned with aggregation resulting from the high concentrations required by other methods. -
Solution
With Dianthus, a high concentration is not required like it is with ITC. And if aggregation happens at low concentration, Dianthus differentiates between binding and aggregation so you get a better understanding of how the molecules behave -
Challenge
Your method requires immobilization which easily disturbs the conformational equilibrium of IDPs. -
Solution
Dianthus measures in solution, so the conformational equilibrium is not at risk of being disrupted as it happens with methods that require immobilization like SPR.
Dianthus is your trusted screening platform from start to finish
Hit Identification
Finding true hits faster is the most important step in making your drug discovery workflow efficient. With Dianthus, you’ll find hits quickly and move on to hit validation confidently, whether it’s fragment-based or small molecule single-dose screening.
Lead Validation
Spend less time sorting through the strong and weak binders. Dianthus generates easy-to-interpret affinity ranking tables and histograms to help you quickly decide on the right candidates and start lead optimization sooner.
Lead Optimization
Once validation is complete, it’s time to improve target specificity, selectivity, and potency. Use Dianthus to verify that binding affinities remain strong. This, combined with your ADME, toxicity, and PK/PD results, ensures you’re developing the best drug candidates.
Validate hits with a platform that offers two biophysical technologies
Orthogonal validation with multiple biophysical methods is a must-have for all your primary screening campaigns. It’s not uncommon to find hits with one method that turn out to be aggregators by another. Orthogonal validation de-risks your projects by confirming hits and uncovering compounds with drug-quality potential missed by other methods. Researchers choose Dianthus for orthogonal validation for confirmation and to collect additional compound attributes for increased confidence in knowing which hits to move to the hit-to-lead step.
Ensure your success with these two biophysical modalities
Measuring challenging interactions between various types of molecules at times requires a different approach. So having two different modalities in the same instrument helps you cover all the types of interactions you encounter. Dianthus comes with two biophysical modalities — Spectral Shift and Temperature Related Intensity Change (TRIC) — that help you measure the strength of your challenging molecular interactions.
-
Spectral Shift
-
TRIC
Spectral Shift
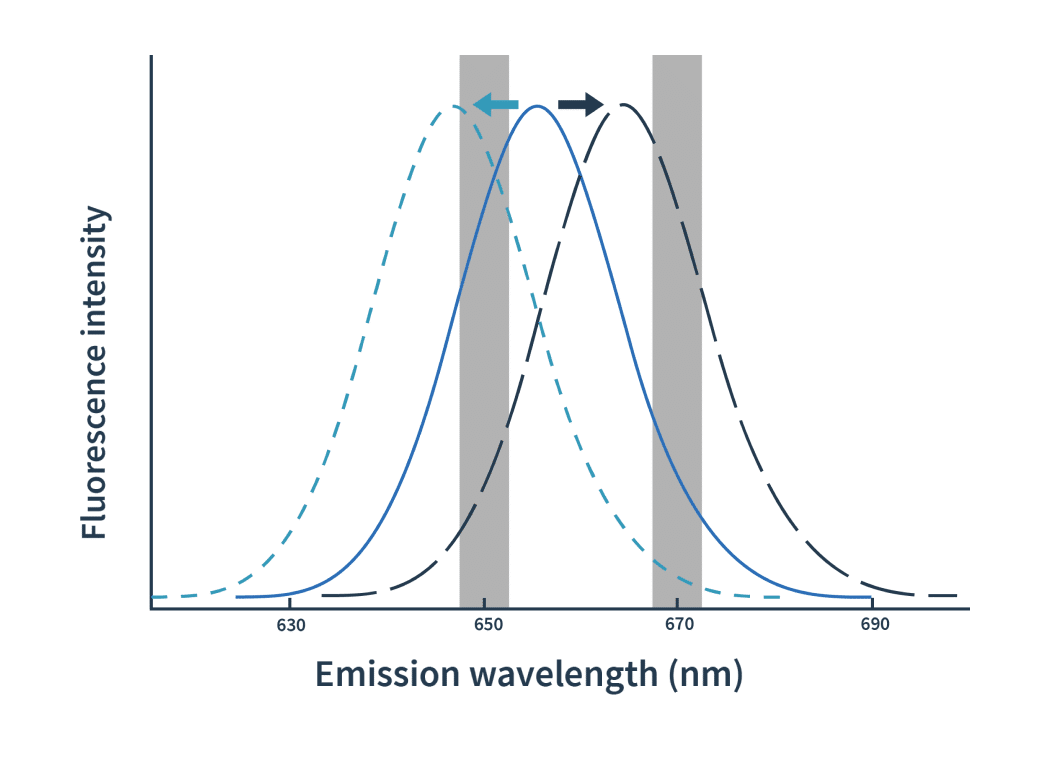
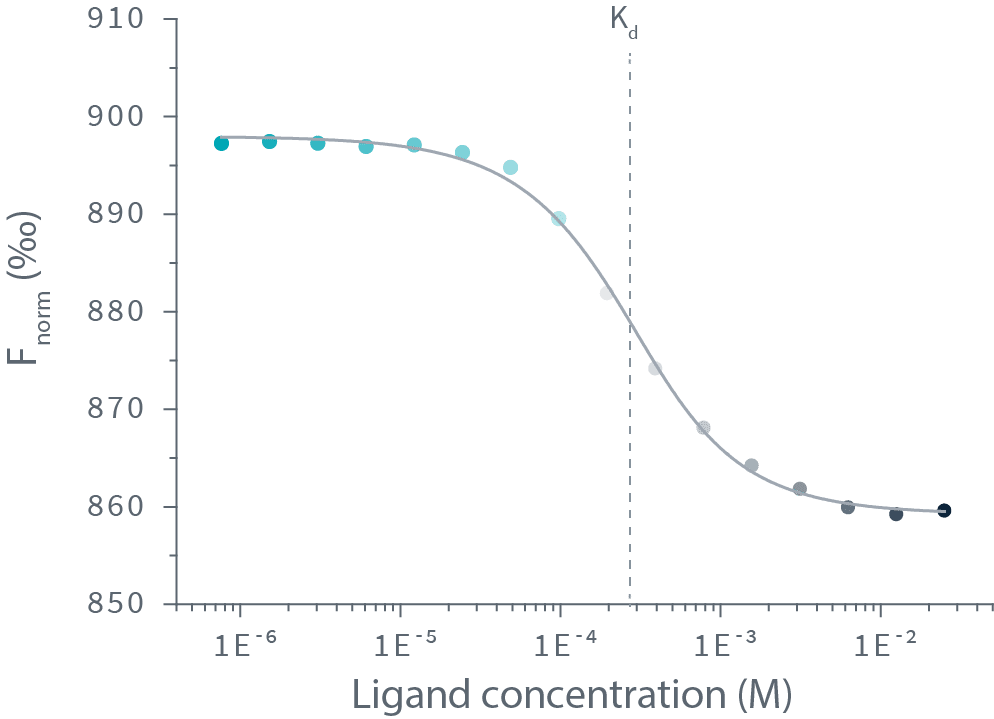
Dianthus is the first affinity screening platform to use Spectral Shift. The notion of spectral shift isn’t new, but Dianthus is the first platform that uses it to derive affinity constants.
The process is actually pretty simple: label one binding partner with a fluorophore, then mix a fixed amount of it with a dilution series of the ligand. After excitation at 590nm of the mix, the binding events are detected as shifts of spectra towards either blue or red. The detection of spectral shifts is achieved through dual-wavelength detection at exactly 650nm and 670nm in an isothermal environment — this makes Dianthus so precise and able to detect very subtle shifts.
Then the Kd is calculated by plotting the ratios of the fluorescence intensities from the two wavelengths against the ligand concentration.
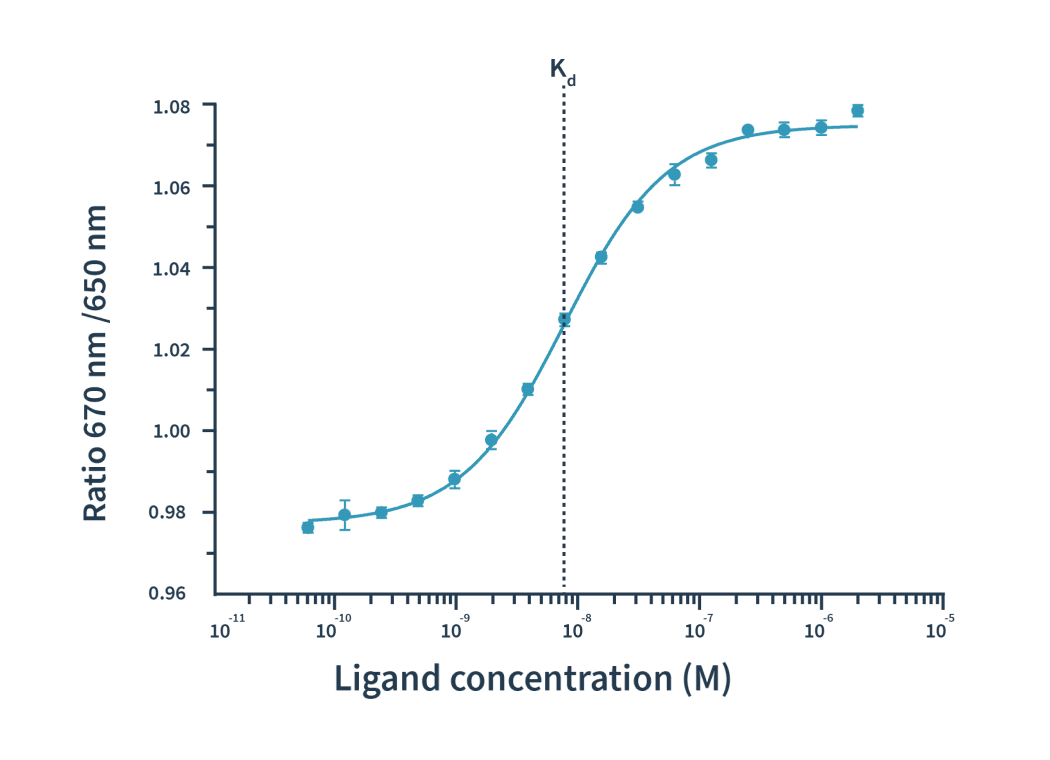
TRIC
Use TRIC — a proven technology that’s been around for over 10 years that compliments Spectral Shift
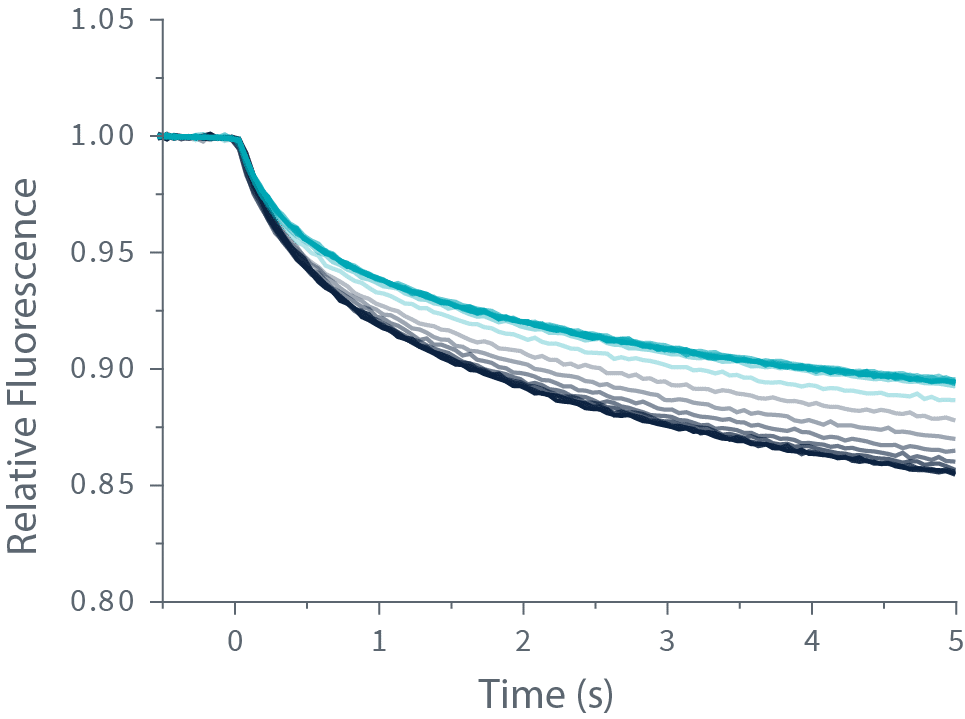
Quantifying molecular interactions with TRIC is done by labeling your target molecule with a fluorescent dye and mixing it with your ligand. Then, a very precise and brief laser-induced temperature change is applied, which amplifies the variation in fluorescence intensity caused by the ligand binding to your target.
The change in fluorescence is measured and plotted against the ligand concentration to obtain the dissociation constant or Kd.

Integrate Dianthus into your existing automation solution
Dianthus is a plate-based, microfluidics-free screening platform that easily integrates into any automated setup via gRPC framework. And with no regular maintenance, your projects don’t get delayed due to downtime. Dianthus is ready whenever you need it — non-stop, 24/7.
Decide which hits are worth moving forward with
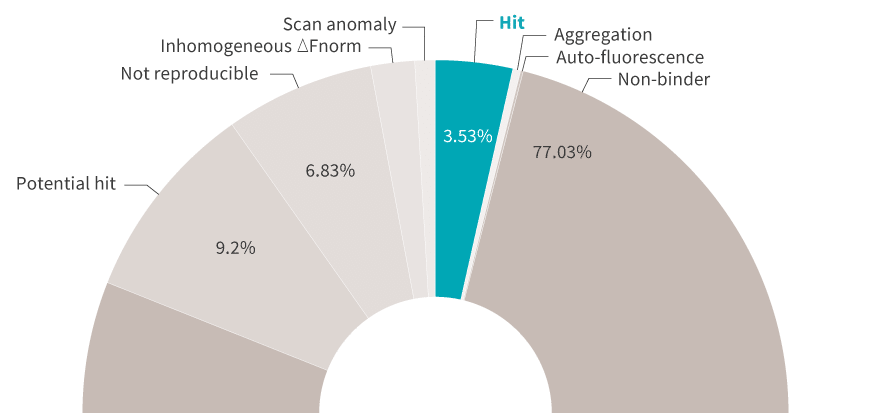
Generating results is great, but getting automated, actionable insights from your results is even better. DI.Screening Analysis software gives you screening summaries as well as easy-to-interpret ranking tables and histograms. And because your Kds are calculated from data with high signal-to-noise ratios, you decide with certainty which candidates are worth moving forward with.
Get consistent results with high-quality consumables

Get consistent results with high-quality consumables
Get the high quality and consistency you expect from consumables when finding and validating hits. Dianthus 384-well plates have all the features needed for the best performance and user experience:
- They are manufactured with a proprietary coating that prevents protein from sticking to the wells
- They go through rigorous QC testing to ensure consistency from well to well
- They have a barcode that helps you track your assay and data back to a specific plate.
You’ll also want to use a labeling kit to label your proteins or peptides with fluorophores selected for their sensitivity to binding events — so you get the best results with Spectral Shift or TRIC.