Prometheus Panta C
Precision that Powers Biopharma Breakthroughs.
From preclinical development to commercial manufacturing.

Introducing Prometheus Panta C
Prometheus Panta C generates high-quality protein stability and aggregation data with the precision and efficiency needed throughout the drug development process — from preclinical research to commercial manufacturing.
By combining four advanced optical technologies, Panta C enables streamlined developability assessment, process monitoring, and characterization of protein identity — all while minimizing sample volume and offering features that support data integrity and 21 CFR Part 11 compliance.
How Prometheus Panta C benefits your workflow
Precision
With advanced optical technology, get high-quality, precise stability data that thoroughly characterize and identify your biologics. Develop fundamental insights to build on in later stages.
Reliability
De-risk your asset and your process with a robust instrument that delivers a repeatable output, and information that you can trust. Don’t lose time redoing experiments or questioning the validity of your data.
Efficiency
Save time and sample volume by measuring multiple stability parameters in parallel, in less than two hours. Be up and running within a day, ready to develop meaningful data with an easy workflow comprising few steps.
Software that enables compliance
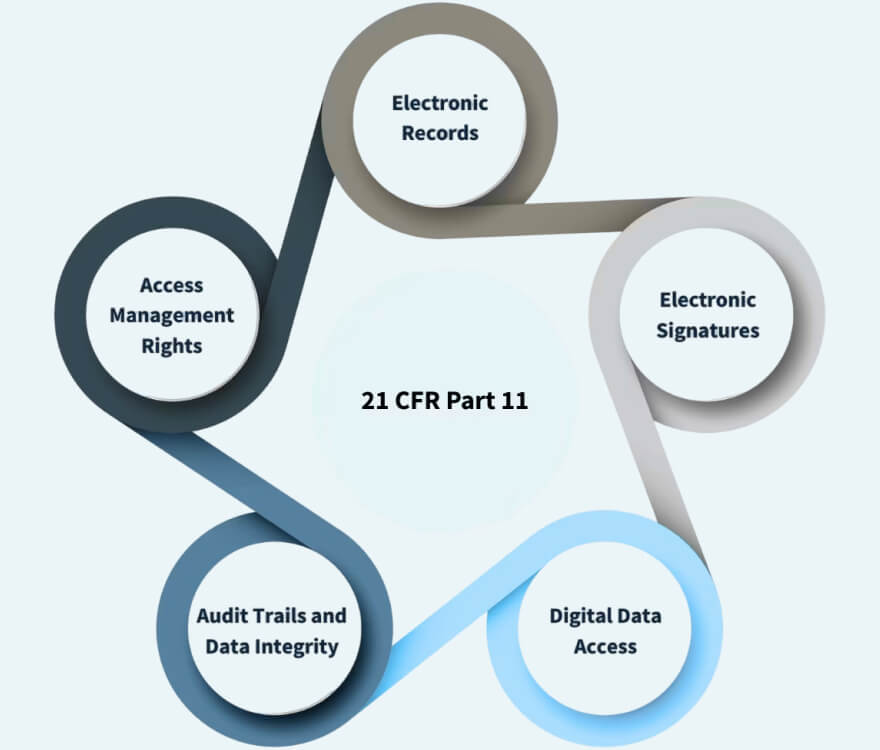
With just a few clicks, PR. Panta C Control Software lets you design and execute single and multi-parameter stability experiments in compliance with 21 CFR Part 11 regulations.
In accordance with regulatory requirements, the Software facilitates the separation of tasks by providing five distinct user access groups, each with predefined permissions: Operator, Reviewer, Supervisor, Administrator, and Service Engineer.
The software is preconfigured to perform two types of experiments:
1) Thermal unfolding measurements, including the acquisition of data from nanoDSF, DLS and Backreflection technologies in parallel, across a thermal ramp.
2) Particle sizing analysis using isothermal DLS data acquisition.
“Prometheus Panta C is really a great workhorse. Working in regulated environment, you can get so much out of it. In addition to ID-testing of Drug Substance, we use it to assess the thermal stability of our Drug Product during process manufacturing and also during process characterization, to check the impact of several stress factors on our Drug Product.”

Johnson and Johnson Innovative Medicine.
Dr. Marius Müller, Team Lead Fill & Finish.
Where Prometheus Panta C is making a difference
Developability assessment
Characterize thermal and colloidal stability to select protein constructs for further development. Screen out poor candidates with undesirable properties early, to minimize development and commercialization risk of your program.
CMC analytical testing
Strengthen the CMC package for your biopharmaceutical in all phases of drug development, from preclinical to commercialization. Track process performance and molecule behavior with high-quality data for your records.
Identification and comparability
Compare molecules to their physiological counterparts or other relevant reference products. Develop algorithms for identification at various stages and establish protein fingerprints using thermal unfolding curves.
Spotlight on biologics ID-testing with Prometheus Panta C
See how Johnson and Johnson Innovative Medicine implemented nanoDSF to efficiently perform identifiy testing of biologics drug substance.
In this video, Dr. Marius Müller from J&J Innovative Medicine describes how he successfully performs ID testing of Drug Substance with Prometheus Panta C. You’ll learn about:
- nanoDSF, a high throughput label free technique that assesses protein stability
- How to use nanoDSF to confirm product identity during biomanufacturing QC
- How this compares to current ID-testing methods
“Prometheus Panta C marks a major milestone in expanding our optical technologies into regulated workflows. This pioneering system enables companies to precisely characterize and identify proteins from preclinical through to commercial manufacturing. Our vision is to make every disease treatable – and this is a step toward that future.”

NanoTemper Technologies.
Dr. Stefan Duhr, CEO and Founder.